Young Researcher Paper Award 2025
🥇Winners
🥇Winners
Print: ISSN 0914-4935
Online: ISSN 2435-0869
Sensors and Materials
is an international peer-reviewed open access journal to provide a forum for researchers working in multidisciplinary fields of sensing technology.
Online: ISSN 2435-0869
Sensors and Materials
is an international peer-reviewed open access journal to provide a forum for researchers working in multidisciplinary fields of sensing technology.
Tweets by Journal_SandM
Sensors and Materials
is covered by Science Citation Index Expanded (Clarivate Analytics), Scopus (Elsevier), and other databases.
Instructions to authors
English 日本語
Instructions for manuscript preparation
English 日本語
Template
English
Publisher
MYU K.K.
Sensors and Materials
1-23-3-303 Sendagi,
Bunkyo-ku, Tokyo 113-0022, Japan
Tel: 81-3-3827-8549
Fax: 81-3-3827-8547
MYU Research, a scientific publisher, seeks a native English-speaking proofreader with a scientific background. B.Sc. or higher degree is desirable. In-office position; work hours negotiable. Call 03-3827-8549 for further information.

MYU Research
(proofreading and recording)

MYU K.K.
(translation service)

The Art of Writing Scientific Papers
(How to write scientific papers)
(Japanese Only)
is covered by Science Citation Index Expanded (Clarivate Analytics), Scopus (Elsevier), and other databases.
Instructions to authors
English 日本語
Instructions for manuscript preparation
English 日本語
Template
English
Publisher
MYU K.K.
Sensors and Materials
1-23-3-303 Sendagi,
Bunkyo-ku, Tokyo 113-0022, Japan
Tel: 81-3-3827-8549
Fax: 81-3-3827-8547
MYU Research, a scientific publisher, seeks a native English-speaking proofreader with a scientific background. B.Sc. or higher degree is desirable. In-office position; work hours negotiable. Call 03-3827-8549 for further information.

MYU Research
(proofreading and recording)

MYU K.K.
(translation service)

The Art of Writing Scientific Papers
(How to write scientific papers)
(Japanese Only)
Sensors and Materials, Volume 37, Number 4(3) (2025)
Copyright(C) MYU K.K.
Copyright(C) MYU K.K.
|
pp. 1527-1535
S&M4002 Research Paper of Special Issue https://doi.org/10.18494/SAM5053 Published: April 22, 2025 Syngas Formation by Microwave-induced Co–Mo and Ni–Mo Catalysts [PDF] Chih Ju G. Jou and Chien Li Lee (Received April 1, 2024; Accepted June 11, 2024) Keywords: Ni–Mo catalyst, Co–Mo catalyst, syngas, microwave
In this study, the microwave-induced Co–Mo and Ni–Mo catalysts were combined with a high-dielectric-constant medium to absorb microwave energy and convert it into the heat energy required for syngas production. The microwave conditions were a microwave power of 450 W and a total microwave time of 210 min with the volume ratios CH4/air = 1:2 (partial oxidation) and CH4/CO2 = 2:1 (dry reforming reaction). The results showed that the average yields of H2 and CO were 43 and 35.7% in the partial oxidation reaction with Ni–Mo as catalyst, whereas those in the dry reforming reaction were 44.4 and 24.9%, respectively. On the other hand, with Co–Mo as the catalyst, the average yields of H2 and CO were 50.7 and 35.9% in the partial oxidation reaction, whereas those in the dry reforming reaction were 62.6 and 40.6%, respectively. Owing to the high activity of the Ni–Mo catalyst, it is easier to shield the metal coated on the surface by coke deposition. As the reaction time proceeds, the activity of the Ni–Mo catalyst decreases, and the syngas yield is reduced. Therefore, it is feasible and innovative to combine microwave-induced Ni–Mo and Co–Mo catalysts with a high-dielectric-constant medium to absorb and convert microwave energy into heat energy to produce syngas.
Corresponding author: Chih Ju G. Jou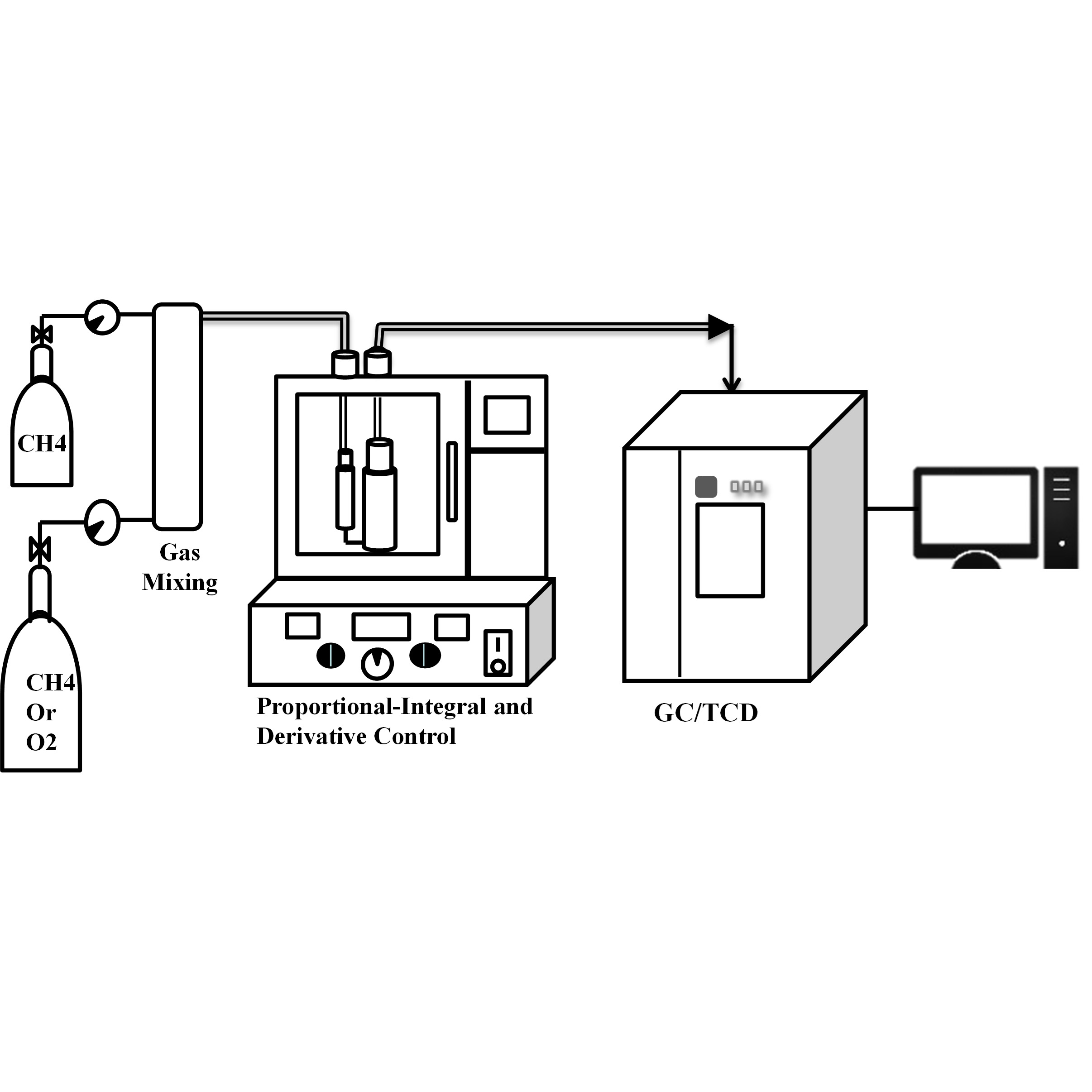  This work is licensed under a Creative Commons Attribution 4.0 International License. Cite this article Chih Ju G. Jou and Chien Li Lee, Syngas Formation by Microwave-induced Co–Mo and Ni–Mo Catalysts, Sens. Mater., Vol. 37, No. 4, 2025, p. 1527-1535. |
Forthcoming Regular Issues
Forthcoming Special Issues
Special Issue on Novel Sensors, Materials, and Related Technologies on Artificial Intelligence of Things Applications
Guest editor, Teen-Hang Meen (National Formosa University), Wenbing Zhao (Cleveland State University), and Cheng-Fu Yang (National University of Kaohsiung)
Call for paper
Special Issue on Advanced GeoAI for Smart Cities: Novel Data Modeling with Multi-source Sensor Data
Guest editor, Prof. Changfeng Jing (China University of Geosciences Beijing)
Call for paper
Special Issue on Mobile Computing and Ubiquitous Networking for Smart Society
Guest editor, Akira Uchiyama (The University of Osaka) and Jaehoon Paul Jeong (Sungkyunkwan University)
Call for paper
Special Issue on Advanced Materials and Technologies for Sensor and Artificial- Intelligence-of-Things Applications (Selected Papers from ICASI 2026)
Guest editor, Sheng-Joue Young (National Yunlin University of Science and Technology)
Conference website
Call for paper
Special Issue on Innovations in Multimodal Sensing for Intelligent Devices, Systems, and Applications (submission closed)
Guest editor, Jiahui Yu (Research scientist, Zhejiang University), Kairu Li (Professor, Shenyang University of Technology), Yinfeng Fang (Professor, Hangzhou Dianzi University), Chin Wei Hong (Professor, Tokyo Metropolitan University), Zhiqiang Zhang (Professor, University of Leeds)
Call for paper
Special Issue on Biosensing Devices
Guest editor, Kiyotaka Sasagawa (Nara Institute of Science and Technology)
Call for paper
-
For more information of Special Issues (click here)
-
Special Issue on Multisource Sensors for Geographic Spatiotemporal Analysis and Social Sensing Technology Part 5
- Accepted papers (click here)
- High-precision Autonomous Driving Map Quality Inspection Indicator System and Evaluation Method
Chengcheng Li, Ming Dong, Hongli Li, Xunwen Yu, Yongxuan Liu, and Chong Zhang
- High-precision Autonomous Driving Map Quality Inspection Indicator System and Evaluation Method
- Accepted papers (click here)
- Voltage Reflex and Equalization Charger for Series-connected Batteries
Cheng-Tao Tsai and Jia-Wei Lin
- Voltage Reflex and Equalization Charger for Series-connected Batteries
- Accepted papers (click here)
- Design and Development of a Fuzzy-logic-based Long-range Aquaculture System
Sheng-Tao Chen and Tai-I Chou
- Design and Development of a Fuzzy-logic-based Long-range Aquaculture System
Guest editor, Prof. Bogang Yang (Beijing Institute of Surveying and Mapping) and Prof. Xiang Lei Liu (Beijing University of Civil Engineering and Architecture)
Special Issue on Materials, Devices, Circuits, and Analytical Methods for Various Sensors (Selected Papers from ICSEVEN 2025)
Guest editor, Chien-Jung Huang (National University of Kaohsiung), Mu-Chun Wang (Minghsin University of Science and Technology), Shih-Hung Lin (Chung Shan Medical University), Ja-Hao Chen (Feng Chia University)
Conference website
Call for paper
Special Issue on Sensing and Data Analysis Technologies for Living Environment, Health Care, Production Management, and Engineering/Science Education Applications (2025)
Guest editor, Chien-Jung Huang (National University of Kaohsiung), Rey-Chue Hwang (I-Shou University), Ja-Hao Chen (Feng Chia University), Ba-Son Nguyen (Lac Hong University)
Call for paper
Special Issue on Advances in Sensors and Computational Intelligence for Industrial Applications
Guest editor, Chih-Hsien Hsia (National Ilan University)
Call for paper
Special Issue on AI-driven Sustainable Sensor Materials, Processes, and Circular Economy Applications
Guest editor, Shih-Chen Shi (National Cheng Kung University) and Tao-Hsing Chen (National Kaohsiung University of Science and Technology)
Call for paper
Special Issue on Intelligent Sensing and AI-driven Optimization for Sustainable Smart Manufacturing
Guest editor, Cheng-Chi Wang (National Sun Yat-sen University)
Call for paper
- Accepted papers (click here)
Copyright(C) MYU K.K. All Rights Reserved.
