Young Researcher Paper Award 2025
🥇Winners
🥇Winners
Print: ISSN 0914-4935
Online: ISSN 2435-0869
Sensors and Materials
is an international peer-reviewed open access journal to provide a forum for researchers working in multidisciplinary fields of sensing technology.
Online: ISSN 2435-0869
Sensors and Materials
is an international peer-reviewed open access journal to provide a forum for researchers working in multidisciplinary fields of sensing technology.
Tweets by Journal_SandM
Sensors and Materials
is covered by Science Citation Index Expanded (Clarivate Analytics), Scopus (Elsevier), and other databases.
Instructions to authors
English 日本語
Instructions for manuscript preparation
English 日本語
Template
English
Publisher
MYU K.K.
Sensors and Materials
1-23-3-303 Sendagi,
Bunkyo-ku, Tokyo 113-0022, Japan
Tel: 81-3-3827-8549
Fax: 81-3-3827-8547
MYU Research, a scientific publisher, seeks a native English-speaking proofreader with a scientific background. B.Sc. or higher degree is desirable. In-office position; work hours negotiable. Call 03-3827-8549 for further information.

MYU Research
(proofreading and recording)

MYU K.K.
(translation service)

The Art of Writing Scientific Papers
(How to write scientific papers)
(Japanese Only)
is covered by Science Citation Index Expanded (Clarivate Analytics), Scopus (Elsevier), and other databases.
Instructions to authors
English 日本語
Instructions for manuscript preparation
English 日本語
Template
English
Publisher
MYU K.K.
Sensors and Materials
1-23-3-303 Sendagi,
Bunkyo-ku, Tokyo 113-0022, Japan
Tel: 81-3-3827-8549
Fax: 81-3-3827-8547
MYU Research, a scientific publisher, seeks a native English-speaking proofreader with a scientific background. B.Sc. or higher degree is desirable. In-office position; work hours negotiable. Call 03-3827-8549 for further information.

MYU Research
(proofreading and recording)

MYU K.K.
(translation service)

The Art of Writing Scientific Papers
(How to write scientific papers)
(Japanese Only)
Sensors and Materials, Volume 36, Number 8(1) (2024)
Copyright(C) MYU K.K.
Copyright(C) MYU K.K.
|
pp. 3201-3210
S&M3727 Research Paper of Special Issue https://doi.org/10.18494/SAM4636 Published: August 2, 2024 Improved Substrate Specificity of Phenylalanine Dehydrogenase for L-Phenylalanine Sensor [PDF] Moemi Tatsumi, Kazutoshi Takahashi, Hiroki Yamaguchi, Uno Tagami, Hiroshi Miyano, Toshimi Mizukoshi, and Masayuki Sugiki (Received September 1, 2023; Accepted April 18, 2024) Keywords: L-phenylalanine, phenylalanine dehydrogenase, amino acid analysis, colorimetry, enzyme-based biosensors
L-Phenylalanine (L-Phe) is one of the essential amino acids and is important for the human body because it is used in the biosynthesis of neurotransmitters. Phenylalanine dehydrogenase (PheDH) has been studied as a material for the L-Phe sensor. In particular, the accurate and simple determination of L-Phe concentrations in human samples is needed for the diagnosis and treatment of patients with phenylketonuria. Thermostable PheDH from the bacterium Thermoactinomyces intermedius is suitable as a sensor material; however, it is reactive to not only L-Phe but also L-tyrosine (L-Tyr). Thus, we attempted to improve its substrate specificity for the accurate measurement of L-Phe. In this study, we developed the PheDH mutant with improved substrate specificity by amino acid substitution. The PheDH mutant, in which the 290th asparagine residue was substituted with aspartic acid (N290D), is considered to form a new interaction in the substrate binding site and has a markedly reduced reactivity with L-Tyr compared with the wild-type PheDH; the relative activities (L-Tyr/L-Phe) of the wild-type PheDH and the PheDH N290D mutant were 64.0% and 1.5%, respectively. An enzymatic assay using this mutant was established to quantify the L-Phe concentration in human plasma. The relative errors were −10.3% to 7.4% when compared with the quantitative value determined by high-performance liquid chromatography/electrospray ionization mass spectrometry. Furthermore, the immobilized mutant showed a concentration-dependent reactivity with L-Phe and no reactivity with L-Tyr, which indicate its potential use as a sensor material.
Corresponding author: Moemi Tatsumi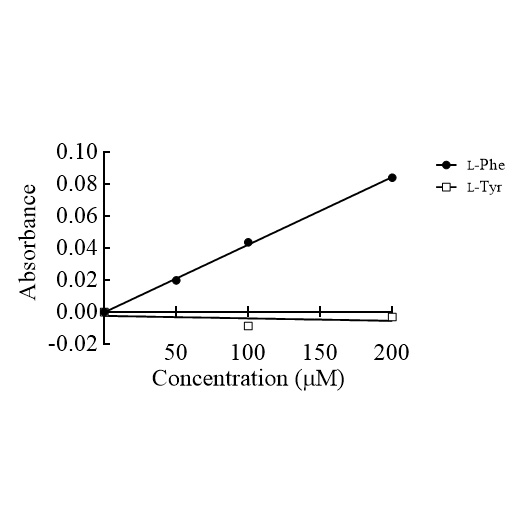  This work is licensed under a Creative Commons Attribution 4.0 International License. Cite this article Moemi Tatsumi, Kazutoshi Takahashi, Hiroki Yamaguchi, Uno Tagami, Hiroshi Miyano, Toshimi Mizukoshi, and Masayuki Sugiki, Improved Substrate Specificity of Phenylalanine Dehydrogenase for L-Phenylalanine Sensor, Sens. Mater., Vol. 36, No. 8, 2024, p. 3201-3210. |
Forthcoming Regular Issues
Forthcoming Special Issues
Special Issue on Novel Sensors, Materials, and Related Technologies on Artificial Intelligence of Things Applications
Guest editor, Teen-Hang Meen (National Formosa University), Wenbing Zhao (Cleveland State University), and Cheng-Fu Yang (National University of Kaohsiung)
Call for paper
Special Issue on Mobile Computing and Ubiquitous Networking for Smart Society
Guest editor, Akira Uchiyama (The University of Osaka) and Jaehoon Paul Jeong (Sungkyunkwan University)
Call for paper
Special Issue on Advanced Materials and Technologies for Sensor and Artificial- Intelligence-of-Things Applications (Selected Papers from ICASI 2026)
Guest editor, Sheng-Joue Young (National United University)
Conference website
Call for paper
Special Issue on Innovations in Multimodal Sensing for Intelligent Devices, Systems, and Applications
Guest editor, Jiahui Yu (Research scientist, Zhejiang University), Kairu Li (Professor, Shenyang University of Technology), Yinfeng Fang (Professor, Hangzhou Dianzi University), Chin Wei Hong (Professor, Tokyo Metropolitan University), Zhiqiang Zhang (Professor, University of Leeds)
Call for paper
Special Issue on Advanced Materials and Technologies for Sensor and Artificial- Intelligence-of-Things Applications (Selected Papers from ICASI 2025)
Guest editor, Sheng-Joue Young (National United University)
Conference website
Call for paper
Special Issue on Multisource Sensors for Geographic Spatiotemporal Analysis and Social Sensing Technology Part 5
Guest editor, Prof. Bogang Yang (Beijing Institute of Surveying and Mapping) and Prof. Xiang Lei Liu (Beijing University of Civil Engineering and Architecture)
-
For more information of Special Issues (click here)
-
Special Issue on Advanced GeoAI for Smart Cities: Novel Data Modeling with Multi-source Sensor Data
- Accepted papers (click here)
- Voltage Reflex and Equalization Charger for Series-connected Batteries
Cheng-Tao Tsai and Jia-Wei Lin
- Voltage Reflex and Equalization Charger for Series-connected Batteries
- Accepted papers (click here)
- Design and Development of a Fuzzy-logic-based Long-range Aquaculture System
Sheng-Tao Chen and Tai-I Chou
- Design and Development of a Fuzzy-logic-based Long-range Aquaculture System
Guest editor, Prof. Changfeng Jing (China University of Geosciences Beijing)
Call for paper
Special Issue on Materials, Devices, Circuits, and Analytical Methods for Various Sensors (Selected Papers from ICSEVEN 2025)
Guest editor, Chien-Jung Huang (National University of Kaohsiung), Mu-Chun Wang (Minghsin University of Science and Technology), Shih-Hung Lin (Chung Shan Medical University), Ja-Hao Chen (Feng Chia University)
Conference website
Call for paper
Special Issue on Sensing and Data Analysis Technologies for Living Environment, Health Care, Production Management, and Engineering/Science Education Applications (2025)
Guest editor, Chien-Jung Huang (National University of Kaohsiung), Rey-Chue Hwang (I-Shou University), Ja-Hao Chen (Feng Chia University), Ba-Son Nguyen (Lac Hong University)
Call for paper
Special Issue on Advances in Sensors and Computational Intelligence for Industrial Applications
Guest editor, Chih-Hsien Hsia (National Ilan University)
Call for paper
Special Issue on AI-driven Sustainable Sensor Materials, Processes, and Circular Economy Applications
Guest editor, Shih-Chen Shi (National Cheng Kung University) and Tao-Hsing Chen (National Kaohsiung University of Science and Technology)
Call for paper
Special Issue on Intelligent Sensing and AI-driven Optimization for Sustainable Smart Manufacturing
Guest editor, Cheng-Chi Wang (National Sun Yat-sen University)
Call for paper
- Accepted papers (click here)
Copyright(C) MYU K.K. All Rights Reserved.
